The Art of Engineering
Thousands of mysteries
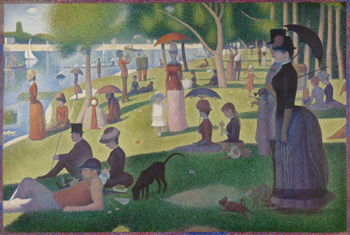 Hanging in the galleries of the Art Institute of Chicago is a mystery - actually, thousands of them. Curators, conservators, and scientists at the Art Institute of Chicago spend countless hours trying to understand each piece of art - when and where it was made, how it was used, how it reflects the historical period, and whether it has changed over time. Their research provides more than an understanding of a specific painting or sculpture; it provides insight into the cultural heritage of a society. Now the Art Institute has a new set of research tools to unlock these mysteries - the faculty expertise and cutting-edge equipment of McCormick.
Hanging in the galleries of the Art Institute of Chicago is a mystery - actually, thousands of them. Curators, conservators, and scientists at the Art Institute of Chicago spend countless hours trying to understand each piece of art - when and where it was made, how it was used, how it reflects the historical period, and whether it has changed over time. Their research provides more than an understanding of a specific painting or sculpture; it provides insight into the cultural heritage of a society. Now the Art Institute has a new set of research tools to unlock these mysteries - the faculty expertise and cutting-edge equipment of McCormick.
The driving forces behind the ongoing collaboration between the two institutions have been Francesca Casadio, Andrew W. Mellon Conservation Scientist at the Art Institute, and Katherine Faber, professor of materials science and engineering at McCormick. With a doctorate in analytical chemistry, Casadio brings a unique background to the Art Institute. When hired in 2003, the Art Institute purchased much of the high-tech equipment she needed, but quickly found that it would be impossible to finance the amount of desired equipment. Casadio connected with Faber and quickly realized that there was 
"Francesca can't reasonably equip her lab with all of the instruments she needs, but there is no need to because of proximity to Northwestern and the shared interests that we are developing," Faber says.
But the relationship between McCormick and the Art Institute goes far beyond shared equipment. "The infusion of science in the museum and stimulation by
The collaboration has been strengthened by a recent grant of $500,000 from the Andrew W. Mellon Foundation - the first multi-year collaboration in conservation science in the nation to involve an art museum and a university. In addition to the Art Institute and Northwestern, the grant funds related efforts at Argonne National Laboratory, a U.S. Department of Energy laboratory located just outside of Chicago.
A unique aspect of the funding is a seminar series that brings experts from academia, museums, and private practice to discuss new techniques in conservation science. During last year's inaugural series, there were three seminars that drew participants from museums across the region, as well as from national institutions such as the Metropolitan Museum of Art.
"The seminar series is really meant to be a cross-fertilization program," says Faber. "It's absolutely essential to making things go well."
And things are going well, as several exciting projects between the Art Institute of Chicago and McCormick demonstrate.
Finding Seurat's light
 Within years of the completion of Georges Seurat's famous A Sunday on La Grande Jatte, the yellow pigment used in the painting began to change. "The yellow, all of the flecks of light in the painting, became very dark and were no longer luminous," explains Kimberly Gray, associate professor of civil and environmental engineering and of materials science and engineering. "It's been a question for over a hundred years - what was it about those pigments that caused them to so rapidly change?"
Within years of the completion of Georges Seurat's famous A Sunday on La Grande Jatte, the yellow pigment used in the painting began to change. "The yellow, all of the flecks of light in the painting, became very dark and were no longer luminous," explains Kimberly Gray, associate professor of civil and environmental engineering and of materials science and engineering. "It's been a question for over a hundred years - what was it about those pigments that caused them to so rapidly change?"
To explore this question, Francesca Casadio sought a suitable light source to determine if the pigments were particularly sensitive to a specific type of light, causing them to change rapidly. The Art Institute didn't have the proper equipment for the experiment, but Gray, whose research interests include photochemistry, did.
Deanna Hurum, a research scientist working in Gray's research lab, worked with Casadio to test pigment changes on a palette of zinc chromate yellow subjected to a xenon arc lamp in order to accelerate the effects that light may have had on the painting.
After analysis of the palette, Casadio determined that the light alone didn't provide cause for the dramatic pigment change seen on Seurat's masterpiece. But, as does often happen in research, the experiment is leading to a series of new, expanded tests.
Nirav Shah, an environmental science major in the Judd A. and Marjorie Weinberg College of Arts of Sciences, is working with Gray and Casadio to build an environmental chamber that will allow the pigment to be tested under different conditions. "We'll try to simulate varying moisture content, acidity, and light and see if some combination of those factors could account for the rapid deterioration of the pigment," Gray says. "We've expanded the experimental design in order to look systematically at the effects of numerous factors and the interaction of those factors that could account for changes in the painting."
The environmental chamber is funded by the Office of Industry Relations, an example of support for the collaboration with the Art Institute extending beyond the Mellon Foundation funding.
To Gray, the collaboration provides an exciting application for the scientific principles used in her research. "It provides interesting opportunities for our students. It brings examples to show how our research and our capabilities can be beneficial for society," she explains. "If you can answer the very basic question, you may find that it has surprising applications. It may have relevance to understanding why a painting faded a hundred years ago or to developing new materials for the future."
Analyzing ancient colors
One of the prize pieces in the Art Institute's collection of Chinese jades is an ancient sculpture of a kneeling figure with its hands bound behind dating back to the Shang Dynasty (1600-1045 BCE). The piece had no known counterpart when it was bequeathed to the Art Institute in 1950 as part of the Edward and Louise B. Sonnenschein Collection, but since 2001 at least 11 stylistically identical pieces have been unearthed in China. Those newly discovered pieces, however, are yellowish gray or dark green in color - unlike the very dark, almost black sculpture at the Art Institute.
"What is of interest was how this piece came to be black," Faber says. "Was the stone actually black? Was this some sort of painting or coating? Or had the object undergone some sort of a burning process to change its color? We decided that once we found what the mineral was, we could address some of these questions."
Faber and Casadio worked with Ariel Knowles (materials science and engineering '05), who used the project for her senior thesis. "As an undergraduate project, it was absolutely terrific," Faber says. "It got her outside of the university and allowed her to use the techniques she's learned in materials science for a project that has to do with cultural heritage, which is certainly an expansion beyond what we normally do in this department."
The research group performed tests at the Art Institute and at Northwestern in order to determine the mineral used in the piece. Casadio performed Raman micro-spectroscopy at the Art Institute, allowing the researchers to gain some insight into the composition of the material, as well as
Prior to bringing the piece to Northwestern for the next round of tests, it was of key importance to ensure the careful handling of the sculpture in the laboratory. In order to properly adjust the instrumentation, Knowles built a plaster model of the piece. The model allowed the group to design a fixture to ensure the security of the piece during testing. The team brought the piece to Northwestern for two days of analysis, including x-ray diffraction to determine the composition and structure of the material and electron microscopy to perform topological studies and chemical analysis of the piece.
The research group was able to determine the composition of the piece from the first round of testing. They were also able to determine that there was no paint on the piece, eliminating one possible cause of the distinctive color.
After acquiring samples of similar minerals, Knowles subjected each sample to a series of heating studies to determine if
In addition to heating the samples, the research group polished and treated each one with Japan wax, residues of which were found on the surface of the kneeling figure. Waxing is a common treatment used by collectors of Chinese jades and other hard stones to enhance an item's appearance. Both treatments were representative of changes to the artifact that may have been caused by collectors after the creation of the piece.
Comparing the color changes, the research presented some likely factors that contributed to the current appearance of the piece. "At the end, we feel reasonably comfortable saying that the piece could have gone through some heating, because we know that we can get color changes to very dark
The end result addresses the unique challenge facing conservation science. Until recently, the care and handling of pieces have rarely been documented, making it difficult to discern the aspects of a piece bestowed by the original artist from changes resulting from collectors and environmental factors. Though it is impossible to provide a decisive answer for a piece such as the jade studied in this project, the scientific analysis, coupled with comparable archaeological data, provides additional insight into the context and provenance of the piece.
Revealing an artist's revisions
 Jack Tumblin has turned a digital camera, laptop computer, and a disco light into a powerful tool for analyzing art.
Jack Tumblin has turned a digital camera, laptop computer, and a disco light into a powerful tool for analyzing art.
Tumblin, assistant professor of electrical engineering and computer science, attended one of the seminars sponsored by the Art Institute and Northwestern, where he met Francesca Casadio, Stephanie D'Alessandro, associate curator of modern painting and sculpture at the Art Institute, and Kristin Lister, paintings conservator. The group discussed challenges facing an upcoming project (set for an exhibition in 2009), which started Tumblin thinking about how his work might be able to complement the Art Institute's research.
The project involved pentimenti (Italian for regrets or
One technique often used to analyze surface detail is raking-angle light, where a light is cast at a low angle, resulting in increased visibility of subtle variations in texture. The problem with raking-angle light is that the variations in texture change based on the position of both the light and the viewer. At different positions, some changes in the texture are highlighted, while others are hidden from view.
Traditional photography catches only one angle of one view, often hiding some of the details within the texture. Using a computer-controlled camera and light (which comes in the form of an off-the-shelf disco light), Tumblin and his students are able to create a digital file that combines the various angles from which a piece can be viewed or lit. The result is an abstract data type that can be relit, giving the user the ability to see a level of detail that would be lost in a typical film-based photograph. This new tool allows users to capture the type of data that is processed by a human eye.
"This is a new way of measuring very subtle surface variations of a painting that's independent of the painting color," Tumblin explains. "We're able to combine this data with infrared imaging and X-rays to get a very rich data set, so we can pull out features people couldn't see before."
Tumblin's device was used on Picasso's Untitled (Man with Moustache, Buttoned Vest, and Pipe), and has initially found surprising results. "The device made it very vivid and clear in the raw data that originally the man was wearing a bowler hat," Tumblin explains. "You can see where Picasso changed his mind."
Tumblin's device and related work may have far-reaching implications for museum science. He recently received National Science Foundation funding to work on the application of the device with the Field Museum. In the long term, Tumblin hopes to create a better way for museums to share their collections with the general public. "I think that one of the best things that we can do as computer graphics people is to make a great improvement to photography, to get away from film and filmlike thinking, where we're just recording the focal plane intensities," he explains. "Instead we can say this is a computational data-gathering device. We want to compute all of the visually important information with our cameras."
Conquering the challenges of corrosion
 David Dunand, professor of materials science and engineering, worked with Casadio and colleagues of the Art Institute of Chicago to analyze several bronze pieces from the museum's Asian art collection. For Dunand, the project seemed to be a perfect mesh of his current interests and family history. "I'm a metallurgist, and my grandfather was also a metallurgist with a wide range of interests, including archaeological materials. My father was a curator of a small museum in Geneva that specializes in Asian art," he explains. "I've always been very close to Asian art. Even though I am a scientist, the other part of my brain is active. For me, art is a very important part of life. It makes us human in our highly technological society - discovery drives the scientist in me, but art allows for emotional expression."
David Dunand, professor of materials science and engineering, worked with Casadio and colleagues of the Art Institute of Chicago to analyze several bronze pieces from the museum's Asian art collection. For Dunand, the project seemed to be a perfect mesh of his current interests and family history. "I'm a metallurgist, and my grandfather was also a metallurgist with a wide range of interests, including archaeological materials. My father was a curator of a small museum in Geneva that specializes in Asian art," he explains. "I've always been very close to Asian art. Even though I am a scientist, the other part of my brain is active. For me, art is a very important part of life. It makes us human in our highly technological society - discovery drives the scientist in me, but art allows for emotional expression."
Dunand and graduate student Marcus Young began the research by studying a 3,000-year-old fragment from an ancient bronze vessel. The original vessel had been acquired as a whole object in the 1940s but was damaged during transport to the West, leading to the discovery that it was indeed a pastiche of soldered fragments (some ancient and others modern and made of pure copper) camouflaged with the application of a green stucco to simulate missing decoration and corrosion products. Once the forgery was discovered, the original fragments were donated to the then-Art Institute curator Charles Fabens Kelley to be used as study pieces.
The research project focused on one of the authentic pieces as an initial test to see if nondestructive experiments using updated analytical methods could determine similar information to the destructive tests previously performed. The fragments provided a testing subject without compromising a piece with much more historical or artistic value.
The team traveled to Argonne National Laboratory to use synchrotron X-rays to examine the thick, bronze pieces and determine their composition and microstructure. Together with Argonne scientists, they worked to determine if the corrosion was natural or forged and if there was any uncorroded bronze core under the corrosion layer in the piece. The team determined that, indeed, there was uncorroded metal in the original fragment, but the real result, according to Dunand, was much more important. "We could show that we could nondestructively probe ancient bronze artifacts not just close to their surface, but in their bulk thanks to the very penetrating X-rays available at Argonne. That allows us to probe other museum-quality objects, which, of course, you would never test destructively." Dunand says.
The group then used the same X-ray techniques to a very valuable piece from the Art Institute collection, a bronze
Dunand and Joseph Lambert, Claire Hamilton Professor of Chemistry in Weinberg College, expect to continue their research on ancient Chinese bronzes, as well as to expand into modern bronze pieces from the 20th century, with the goal of identifying the alloy and patina used in each piece. "We'd like to know if an artist systematically used a certain type of bronze, maybe because of the resulting patina," Dunand explains.
As the research continues, perhaps the biggest challenge is choosing a direction. "There is a huge quantity of objects that could be studied in this way," Dunand says. "The challenge is to pick the right questions and the right objects, because there's a backlog of thousands of years of research."
Pinpointing pigments
 The collaboration between the Art Institute and Northwestern also extends beyond McCormick and into the Weinberg College of Arts and Sciences. Richard Van Duyne, Charles E. and Emma H. Morrison Professor of Chemistry, and graduate student Alyson Whitney are working to determine new methods to identify the pigments used in particular pieces of art. This determination is important: The attributes of a particular pigment affect the conditions needed to preserve a piece, give insight into the artist's methods, and provide another method to verify a piece's authenticity.
The collaboration between the Art Institute and Northwestern also extends beyond McCormick and into the Weinberg College of Arts and Sciences. Richard Van Duyne, Charles E. and Emma H. Morrison Professor of Chemistry, and graduate student Alyson Whitney are working to determine new methods to identify the pigments used in particular pieces of art. This determination is important: The attributes of a particular pigment affect the conditions needed to preserve a piece, give insight into the artist's methods, and provide another method to verify a piece's authenticity.
Whitney approached Casadio with a desire to combine her chemistry background with her appreciation of art. The two discovered a shared interest in Raman spectroscopy, a method that has proved particularly useful in the study of pigments. This nondestructive method provides a wealth of useful information for inorganic pigments but has significant limitations with organic pigments. When undergoing testing, organic molecules fluoresce, making it impossible to read the Raman scattering used to determine the makeup of a molecule.
Using surface-enhanced Raman spectroscopy (SERS), however, the group has been able to uncover a wealth of new information about these organic pigments. SERS was discovered by Van Duyne in 1977 and is now widely recognized as the most sensitive form of spectroscopy capable of identifying molecules. "If you coat a substrate with a very thin nanometer layer of silver, you will get a beautiful Raman spectrum of the pigment," Van Duyne explains. "The overlayer of silver does two things - it quenches the fluorescence and it amplifies the Raman scattering."
This new application of SERS allows researchers to rapidly determine the molecular "fingerprint" of pigments that haven't yet been studied. The group started with an analysis of red lakes (pigments obtained by precipitating an organic dyestuff onto an inorganic substrate), which are particularly hard to study. "No one has done this before, so we've been developing a library focusing on red pigments," Whitney explains. "We're determining what these red pigments look like with a silver film on them, so then other conservators can use the information."
The development provides many new opportunities to reexamine previously studied pigments. "Because they've basically been impossible to study before, you don't know what kind of dating and authentication information is contained in these organic materials," Van Duyne explains. "We may find new signatures that are characteristic for a particular artist or time period."
